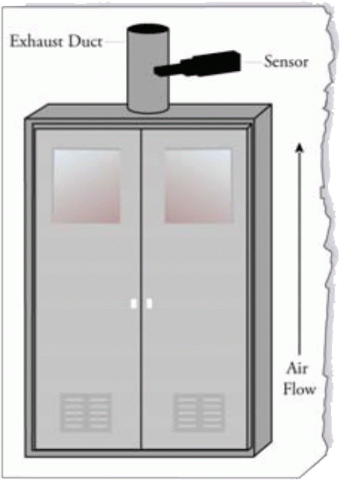
Meeting NFPA Requirements in Ovens & Dryers
Almost all safety authorities require a 4:1 margin of safety below the LFL, based on worst-case conditions. This means that enough dilution air must be used to always maintain a concentration of less than 25% of the LFL, according to the National Fire Protection Association standard NFPA 86.